The vertebrate immune system protects the host from a multitude of potentially pathogenic microorganisms. Although this is commonly thought to be the principal role of the immune system, it is now recognized that its diverse components are deployed in numerous physiological processes, ranging from tissue repair and regeneration to pruning of neurological synapses to conveying information on nutrient availability and metabolic requirements. Disturbances in immune system functions can hence result in a wide variety of diseases, including not only those of the autoimmune and inflammatory variety, but also cardiovascular, metabolic, and psychiatric/neurodegenerative diseases. An additional layer of complexity is provided by the commensal microbiota, whose composition is linked to a wide variety of physiological functions, many of which are conveyed through its effects on the immune system. Our laboratory studies how information from the environment, including microbiota and metabolites, is relayed to cells of the immune system and how this is manifested in homeostatic processes as well as in pathological conditions. Brief summaries of our interests are included below, as are links to lengthier descriptions and relevant publications.
Lineage commitment
A major focus of our group has been on characterizing the cellular interactions and molecular mechanisms involved in the specification of distinct lymphoid lineages during development and in their subsequent functional diversification in peripheral organs. A central unsolved problem in developmental immunology is the mechanism for how progenitor thymocytes branch into either the CD4 (helper and regulatory) or the CD8 (cytotoxic) lineages. We are characterizing the transcriptional regulatory mechanisms involved in lineage specification and in regulation of Cd4 gene expression, with emphasis on the mechanisms for establishing epigenetic programs, e.g. DNA methylation and demethylation and heritable histone marks, in developing T cells. Learn More
Th17, t-regs, & inflammation
Our studies in thymocytes led us to identify the nuclear receptor RORγt as having critical roles in lymphoid development, e.g. survival of immature thymocytes and specification of lymphoid tissue inducer (LTi) cells and type 3 innate lymphoid cells (ILC3), and in the differentiation of multiple types of T cells that produce IL-17, e.g. pro-inflammatory TH17 cells. We are characterizing how RORγt directs the diverse programs of differentiation of lymphoid cells, using biochemical and genomics approaches. We are focusing on how TH17 cells differentiate from naïve CD4+ T cells in vitro and in vivo, in particular on how different signaling inputs result in pathogenic versus non-pathogenic or homeostatic programs. For example, we identified the serum amyloid A (SAA) proteins as upstream regulators of TH17 cell differentiation. In mouse models of inflammatory or autoimmune disease, the SAAs promote the pathogenic functions of TH17 cells. We are currently characterizing the T cell signaling pathways engaged by the SAAs. These studies may provide new therapeutic strategies for the numerous inflammatory diseases mediated by TH17 cells and related IL-17-producing T cells. Learn more
Microbiota-immune system interactions
In the course of elucidating the mechanism of differentiation of Th17 cells, we discovered that those cells were specifically induced when animals were colonized with a defined bacterial species in their intestines. This discovery has led us to investigate how diverse microbial species induce antigen-specific T cells with unique functional programs. We have further learned that microbiota can influence susceptibility to autoimmune diseases and even to behavioral abnormalities in offspring of mothers with activated immune responses. Learn More Elucidation of these mechanisms will help us to understand how normal protective immune responses differ from pathogenic ones that result in inflammation and autoimmune disease, and may thus inform novel therapeutic strategies for multiple disease processes. Learn More
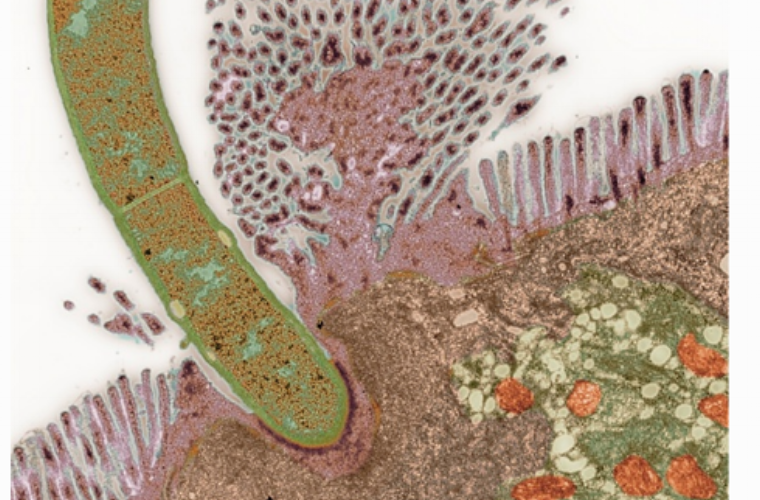
regulation of intestinal homeostasis by the enteric nervous system
Commensal microbes contribute to regulation of intestinal epithelial barrier integrity and function, in large part through modulation of cytokine production by multiple cell types. For example, ILC3 produce IL-22 in response to signals from the microbiota, and this cytokine induces intestinal epithelial cells to produce antimicrobial peptides while inhibiting lipid transport. We found that a subset of ILC3 form cell aggregates that receive direct signals from a subset of enteric neurons producing vasoactive intestinal peptide (VIP) in response to feeding. VIP inhibits IL-22 production by ILC3, resulting in changes in nutrient absorption and enhanced growth of some commensal microbes. We are investigating how VIP neurons are regulated by feeding and also how functions of ILC3 are modulated by the neuropeptide versus signals from commensal bacteria. We are also characterizing other types of enteric neurons and glia in an effort to map their interactions with immune system cells and elucidate their functions. Learn more
microbiota and human disease
How environmental factors, including potentially pathogenic microbes, contribute to immune-mediated pathology remains a largely open question. Multiple studies in animal models have described the effect of the microbiota on a variety of host physiological functions. However, it has been difficult to demonstrate causal links of microbiota with human disease. For example, we showed that new onset rheumatoid arthritis patients are much more likely than healthy subjects to harbor Prevotella copri in their fecal microbiota, but it remains unknown if this bacterial species contributes to disease onset or progression. Recent publications have revealed associations of intestinal microbial communities with responses to cancer “checkpoint” immunotherapy, e.g. anti-CTLA4 or anti-PD-1. The systemic effects of microbiota in autoimmune disease and in anti-tumor immune responses likely reflect related mechanisms. We are thus characterizing human-derived bacterial species and defined bacterial communities that promote pathogenesis in mouse models of autoimmunity or that influence tumor-specific immune responses, with the goal of identifying the relevant cells and molecular pathways targeted by the microbial products. Learn more
mechanisms of hiv pathogenesis
Our laboratory has had a longstanding interest in elucidating the mechanisms by which the Human Immunodeficiency Virus is transmitted and damages the immune system. HIV fuses to target cells after binding to CD4 and CCR5. Infection of T cells is enhanced if HIV first interacts with dendritic cells, which are relatively resistant to infection, but present virus to the target cells. We have employed genetic and cell biological approaches to study the mechanism for enhancement of viral entry by dendritic cells, and have continued to work on developing animal models for eliciting more effective HIV-specific humoral and cellular immunity. We are particularly interested in learning how to harness the intestinal microbiome to enhance anti-viral immune responses and limit viral pathogenesis. Learn More